The CONCORD proposal combines multidisciplinary
approaches aiming at overcoming several limitations regarding immunotherapy of
CLL patients. In detail, It aims to provide an efficient carrier
for mRNA to be used as a safe CAR T-cell therapy, which is an advanced and
novel immunotherapy which is usually applied with virus. Previous approaches to
mRNA as a therapeutic agent have used naked mRNA or polymeric NPs and relied in
short term expression that can work for vaccination purposes but would not be
enough for CAR-T cell anti-tumoral immunotherapy. In detail, the innovation and
added value of the proposal relies in the production of cutting-edge
transfection nanovectors for the sustained release of mRNA inside the cell for
longer therapeutic levels of CAR expression. The advanced cytoplasmic delivery is attributed to the proton
sponge-controlled release. Besides, the vehicle, thanks to its high electronic
density and collective oscillation of the surface electrons, are powerful tools
for imaging, thermal and radio therapy.
One of the main problems of current CAR-T immunotherapy is permanent
alteration of the T cells genotype, even beyond tumor eradication. When solving
this problem with mRNA, activity time
become a major issue. In mammalian cells, mRNA half-lives typically range from
several minutes to 20 hours[1] and
mRNA transfection usually results in a receptor expression of around 20-24h[2].
CAR-19 has already been expressed in T-cells before by means of mRNA
electroporation were an extended expression of CD19 up to 6 days was observed.
It was assumed the unusual receptor half-life is due to their vector and mRNA
production[3].
Other authors reported up to 3 day expression of CD19 in T-cell after mRNA
transfection[4].
Thus combining RNA stability and sustained release it is envisaged that no more
than 4-5 injections would be needed for a complete treatment. Similar
stabilities are expected to be found in our nanocarriers, slow release for
sustained levels of mRNA in the cytosol aimed to extend the half-life of the
CD19 expression to levels that allow a sensible and effective therapeutic
regime.
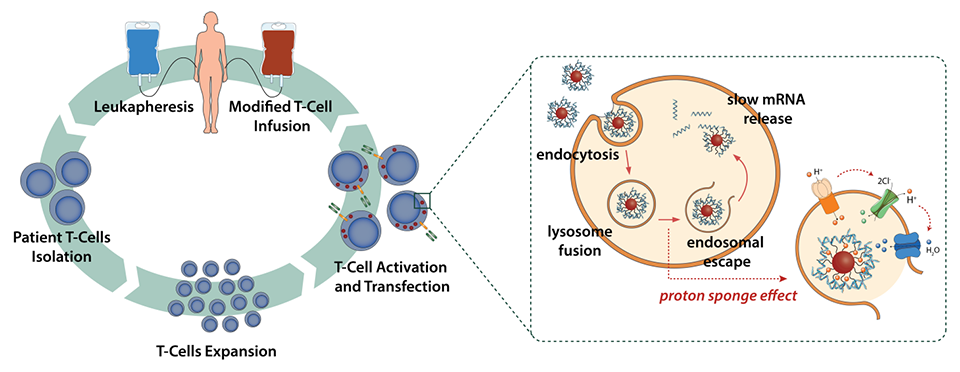
Schematic
representation of the CAR T-cells therapeutic approach (left) and mechanistic
principle of proton sponge – endosomal scape – mRNA delivery for T-cell
modification (right).
SPECIFIC OBJECTIVES
i) To
develop genetic material nanovectors to overcome current polymeric and viral
limitations;
ii) To apply this to
deliver mRNA for transient genetic therapy and promote the synthesis of CAR
T-cells;
iii) To design a CAR T-cell therapy for CLL that is safer and easy
to use and preserves B-cell function for longer term once the patient is cured.
PROJECT STRUCTURE
To achieve
the above detailed objectives, the proposal divides the work in two
different blocs: Nanovector Design Hub (ICN2
and TAU) where the nanovectors are designed, synthesized, functionalized,
loaded with mRNA, characterized and dispersed in biological media, and Nanovector Testing Hub (HCLINC, MNEGRI)
where the molecular mechanisms regulating the
interaction cells-nanovectors, their hazard and risk assessment and the
efficacy and therapeutic power is studied and evaluated.
CONCORD will exploit the endocytosis – proton sponge – endosomal
release route for the cytosol with a work plan
divided in 6 work packages (WPs), covering the whole duration of the project, as
described below in the Gantt chart. The relation between objectives and tasks execution is
not sequential. This implies that some tasks will be carried out in parallel
which translates into short-term results that will be optimized over the entire
project.
WP1
(ICN2) is devoted to the design and
development of the nanovector. The main objective is to design, produce,
characterize and study the physicochemical evolution of the nanovectors in
biological media aiming at overcoming current polymeric and viral
limitations. It will be composed by Au NPs
functionalized with PEI and further loaded with mRNA.
WP2 (TAU) studies the mechanisms of interaction cells-nanovectors
to support its design and refinement. It
will optimize and standardize the
non-viral, efficient and inexpensive approach towards CAR19 expression in
T-cells and their expansion. Mechanistic aspects of the mRNA delivery and
efficacy. Interaction at the molecular level. This will allow to study the
transfection nanovector interaction with biomolecules and cells in view of
refining its design.
WP3 (MNEGRI) is devoted to study hazard and risk assessment of the newly
developed nanovector. For the risk assessment of the nanovector the toxic
potential of the novel products, their components and derivates at all
development stages will be investigated. We plan to use different
cell lines (Jurkat and primary T lymphocytes, but also other human and murine
cancer and normal cells) that will be transfected using the same technology
used for efficacy studies.
WP4 (HCLINIC) will evaluate the efficacy and the
therapeutic power of the nanovector in
vitro and in vivo.
WP5 (ICN2) is devoted to
exploitation, communication and technology transfer
WP6 (ICN2) will address the
project management.
[1] Structural and
Functional Analysis of an mRNP Complex That Mediates the High Stability of Human β-Globin mRNA Mol Cell Biol. 2001 Sep;
21(17): 5879–5888
[2] Transfer
of mRNA encoding recombinant immunoreceptors CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Ther. 2009;16:596–604.
[3] Treatment of Advanced Leukaemia in Mice with mRNA Engineered T Cells Hum Gene Ther. 2011 Dec; 22(12): 1575–1586.
[4] Expression
of CAR in natural killer cells with a regulatory-compliant non-viral method. Cancer Gene Ther. 2010 17:147-54.